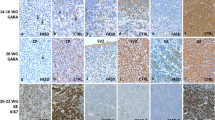
Objectives. To assess the influences of prenatal alcoholization on the formation of various structural components of the brain in human embryos. Materials and methods. The study examined 26 specimens of embryonic material at 8–11 weeks of intrauterine development. Specimens were divided into four subgroups depending on developmental period (Control 1 at 8–9 weeks of development and Control 2 at 10–11 weeks of development) and maternal medical history (presence or absence of a diagnosis of “alcoholism stages I–II” in the medical history). Morphometry was run on Nissl-stained semithin sections. Diameters and areas of each individual tissue element (neuroblasts, glioblasts, vessels of the micricirculatory bed (MCB)) were determined, along with specific areas (the ratio of the total area of the structure under study to the area of the entire section); mean numbers of these structures per unit area of sections were also calculated. Morphometric analysis was run in AxioVision 4.8 (Carl Zeiss, Germany) and statistical analysis of differences between study cohorts used the Mann–Whitney test (differences taken as significant at p < 0.05). Results: As compared with the intact groups, the alcohol groups showed insufficient increases in the area of MCB vessels, combined with compensatory increases in the numbers of vessels per unit area on sections (48.5 and 83.3 μm2, respectively, p < 0.05). Comparison of glioblast sizes in the control and alcohol subgroups at different stages of development (average area 21.3 and 32.1 μm2; 12.9 and 13.3 μm2, respectively) revealed a delay in the increase in size of cellular structures in alcoholized groups at the initial stages, while comparison of data at later periods revealed no significant difference, though there was an increase in the specific number of cells in subgroup A2 (p < 0.05). Neuroblasts also showed a decrease in cell size with increasing development time in both the control and the alcohol subgroups. However, cell size in group A2 was greater than that in group C2, while the number of these cells was smaller (p < 0.05). Conclusions. Alcohol led to changes in the sizes and numbers of neuroblasts, glioblasts, and MCB vessels and, as a consequence, to disproportionality in the development of all brain tissue. Changes progressed with increasing development time.
Similar content being viewed by others
References
Bukiya, A. N. and Dopico, A. M., “Fetal cerebral circulation as target of maternal alcohol consumption,” Alcohol Clin. Exp. Res., 42, No. 6, 1006–1018 (2018).
Joya, X., Garcia-Algar, O., Salat-Batlle, J., et al., “Advances in the development of novel antioxidant therapies as an approach for fetal alcohol syndrome prevention,” Birth Defects Res. A, Clin. Mol. Teratol., 103, No. 3, 163–177 (2015), Epub 2014 Aug 18.
Georgieff, M., Tran, P., and Carlson, E., “Atypical fetal development: Fetal alcohol syndrome, nutritional deprivation, teratogens, and risk for neurodevelopmental disorders and psychopathology,” Dev. Psychopathol., 30, No. 3, 1063–1086 (2018).
Jarmasz, J., Basalah, D., Chudley, A., and Del Bigio, M., “Human brain abnormalities associated with prenatal alcohol exposure and fetal alcohol spectrum disorder,” J. Neuropathol. Exp. Neurol., 76, No. 9, 813–833 (2017).
Treit, S., Zhou, D., Chudley, A., et al., “Relationships between head circumference, brain volume and cognition in children with prenatal alcohol exposure,” PLoS One, 11, No. 2, e0150370 (2016).
Hendrickson, T. J., Mueller, B. A., Sowell, E. R., et al., “Cortical gyrification is abnormal in children with prenatal alcohol exposure,” NeuroImage Clin, 15, 391–400 (2017).
Jégou, S., El Ghazi, F., de Lendeu, P. K., et al., “Prenatal alcohol exposure affects vasculature development in the neonatal brain,” Ann. Neurol., 72, No. 6, 952–60 (2012).
Solonsky, A. V., Logvinov, S. V., and Kutepova, N. A., “Development of brain vessels in human embryos and fetuses in conditions of prenatal exposure to alcohol,” Neurosci. Behav. Physiol., 38, No. 4, 373–376 (2008).
Wilhelm, C. J. and Guizzetti, M., “fetal alcohol spectrum disorders: An overview from the glia perspective,” Front. Integr. Neurosci., 9, 65 (2016).
Goeke, C. M., Hashimoto, J. G., Guizzetti, M., and Vitalone, A., “Effects of ethanol-and choline-treated astrocytes on hippocampal neuron neurite outgrowth in vitro,” Sci. Prog., 104, No. 2, 368504211018943 (2021).
Medina, A. E., “Fetal alcohol spectrum disorders and abnormal neuronal plasticity,” Neuroscientist, 17, No. 3, 274–287 (2011).
Creeley, C. E., Dikranian, K. T., Johnson, S. A., et al., “Alcoholinduced apoptosis of oligodendrocytes in the fetal macaque brain,” Acta Neuropathol. Commun., 1, 23 (2013).
McLachlan, K., Vavasour, I., MacKay, A., et al., “Myelin water fraction imaging of the brain in children with prenatal alcohol exposure,” Alcohol Clin. Exp. Res., 43, No. 5, 833–841 (2019).
Lowery, R. L., Cealie, M. Y., Lamantia, C. E., et al., “Microglia and astrocytes show limited, acute alterations in morphology and protein expression following a single developmental alcohol exposure,” J. Neurosci. Res., 99, No. 8, 2008–2025 (2021).
Collier, A. D., Halkina, V., Min, S. S., et al., “embryonic ethanol exposure affects the early development, migration, and location of hypocretin/orexin neurons in zebrafish,” Alcohol Clin. Exp. Res., 43, No. 8, 1702–1713 (2019).
Lee, S. M., Yeh, P. W. L., and Yeh, H. H., “L-type calcium channels contribute to ethanol-induced aberrant tangential migration of primordial cortical GABAergic interneurons in the embryonic medial prefrontal cortex,” eNeuro, 9, No. 1, ENEURO.0359-21.2021 (2022).
Marguet, F., Friocourt, G., Brosolo, M., et al., “Prenatal alcohol exposure is a leading cause of interneuronopathy in humans,” Acta Neuropathol. Commun., 8, No. 1, 208 (2020).
Farber, N. B., Creeley, C. E., and Olney, J. W., “Alcohol-induced neuroapoptosis in the fetal macaque brain,” Neurobiol. Dis., 40, No. 1, 200–206 (2010).
Raghunathan, R., Liu, C. H., Kouka, A., et al., “Dose-response analysis of microvasculature changes in the murine fetal brain and the maternal extremities due to prenatal ethanol exposure,” J. Biomed. Opt., 25, No. 12, 126001 (2020).
Alarcon-Martinez, L., Yemisci, M., and Dalkara, T., “Pericyte morphology and function,” Histol. Histopathol., 36, No. 6, 633–643 (2021).
Miller, M. W. and Robertson, S., “Prenatal exposure to ethanol alters the postnatal development and transformation of radial glia to astrocytes in the cortex,” J. Comp. Neurol., 337, No. 2, 253–66 (1993).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 123, No. 6, pp. 100–105, June, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Solonsky, A.V., Shumilova, S.N., Potapov, A.V. et al. Structural Changes in Human Brain Tissue in Prenatal Alcoholization at Different Periods of Intrauterine Development. Neurosci Behav Physi 54, 22–26 (2024). https://doi.org/10.1007/s11055-024-01563-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-024-01563-4